
Indeed, the reactions occurring in the oxidative degradation phase during the photo-oxidative degradation of PE are the factors leading to molecular weight reduction. Therefore, studies on the photo-oxidative degradation of PE should focus more on those properties that are favorable for biodegradation and which undoubtedly accelerate the complete degradation of PE microplastics into the water and carbon dioxide. However, most of the polymer fragments eventually become microplastics because they have difficulty meeting the conditions for metabolism by microorganisms before entering the ground, and polyethylene is one of the main sources of microplastics that are causing irreparable damage to freshwater lakes, groundwater, soil, marine ecosystems, and even freshwater animals. These tiny fragments are enriched underground and gradually recognized, digested, and eventually metabolized by microorganisms into carbon dioxide and water. Ideally, PE undergoes photo-oxidative degradation to form tiny fragments with polysaccharide-like structures and molecular weights below 5000 Da. Meanwhile, the β-scission of alkoxy macroradicals and the Norrish reaction of carbonyl products lead to the breakage of the polyethylene main chain, transforming the polyethylene from long-chain macromolecules to short-chain small molecules.
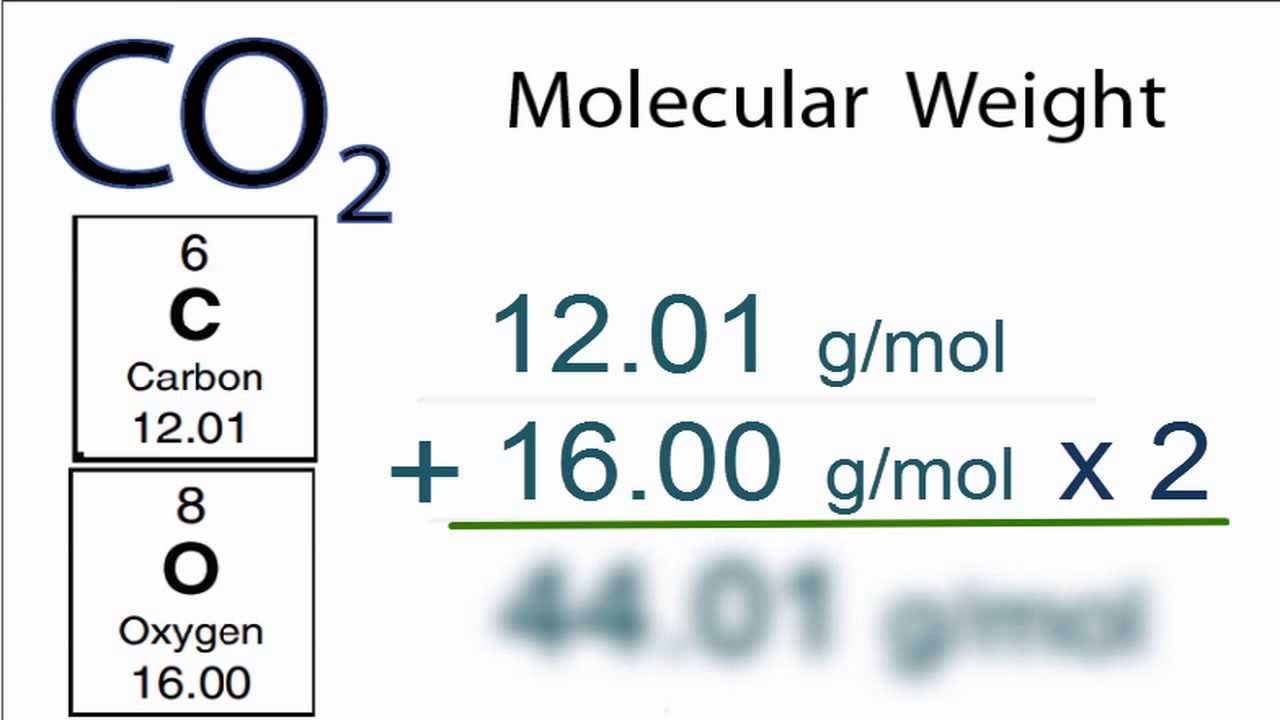
MOLAR MASS OF CO2 SERIES
The series of reactions in the oxidative degradation stage generate oxygen-containing products such as ketones, aldehydes, carboxylic acids, and alcohols, which give the local chain segments of PE a polysaccharide-like structure and provide the possibility of microbial recognition. The reaction of secondary alkyl macroradicals with oxygen causes PE to enter the oxidative degradation stage. Primary alkyl radicals regenerate C-C bonds in situ and emit heat due to site-resistance effects and mobility limitations. The photo-oxidative degradation of PE begins with the photo cracking of the C-H and C-C bonds, generating H radicals, secondary alkyl macroradicals, and primary alkyl macroradicals, as shown in Scheme 1. The mechanism of photo-oxidative degradation of polyethylene was reported in detail by Costa and Bracco. The excellent photodegradation properties of PE/Fe-MMT films will be useful in the design of more environmentally friendly degradable polymers. In addition, Fe-MMT can greatly accelerate the reduction of PE molecular weight into small oxygen-containing molecules as well as induce cracks on the surface of polyethylene films, all of which can accelerate the biodegradation process of polyethylene microplastics.

This new mechanism is an improvement on the existing mechanism of molecular weight reduction during the photo-oxidative degradation of PE. Based on this, it was found that the transfer and coupling of primary alkyl radicals originating from photoinitiation lead to a decrease in the molecular weight of polyethylene, and the kinetic results validate this new mechanism well. A decrease in the molecular weight of polyethylene was also found in the photodegradation phase. The results show the rate of photo-oxidative degradation of each PE/Fe-MMT film is much faster than that of the pure linear low-density polyethylene (LLDPE) film.

The present study aims to investigate the photodegradation of PE/Fe-montmorillonite (Fe-MMT) films, especially molecular weight change. However, the mechanism of molecular weight reduction before oxidative degradation has not been clarified.
The reactions occurring in the oxidative degradation phase during the photo-oxidative degradation of polyethylene (PE) are the factors leading to molecular weight reduction.


 0 kommentar(er)
0 kommentar(er)
